JSK BIOMED Commercializes the World’s First 'Needleless Syringe, Mirajet'
JSK BIOMED, a bio start-up headquartered in Daedeok Innopolis in Daejeon, is garnering attention for commercializing a needleless syringe.
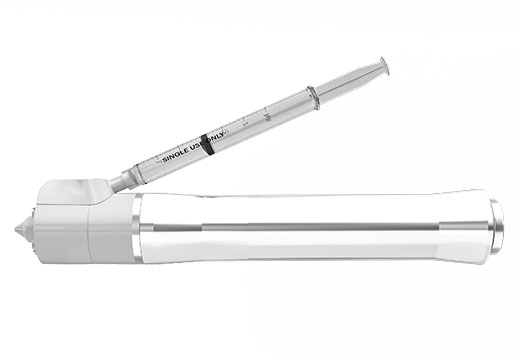
The company announced that it began mass production after being transferred the needleless syringe technology from Seoul National University in 2015 and successfully developed the needleless syringe, 'Mirajet', in 5 years. The needleless syringe has become the world’s first to receive a ‘CE-MDD’ mark under the European Medical Devices Directives.
The CE-MDD stipulates matters related to medical device quality, performance, durability, and safety proposed by the European Union (EU) and CE-MDD is issued only to products that passed all the stringent conformity requirements and judgment criteria. All medical devices exported to and sold in Europe must obtain the certification. JSK BIOMED, which has acquired the CE-MDD for Mirajet, can now export to the 27 member states of EU and the Middle East, Asia, and Latin America, which recognize the CE mark. The bio start-up is also preparing to obtain the US FDA approval.
'Mirajet' is considered a suitable drug delivery system for skin care, as it uses the powerful energy of laser to disperse the drug instantaneously for skin penetration, which is a different mechanism from the existing pneumatic needleless injection systems. Also, laser emission occurs at a rate of 40 times per second, and such high speed makes the procedure nearly painless for patients.
Jeon Jin-woo, the CEO of JSK BIOMED, stated that the needle-free 'Mirajet' will be a perfect alternative for patients with trypanophobia and that the global market for needleless syringes will grow to more than KRW 3 trillion in size.
It is projected that 'Mirajet' will be in great demand from esthetic and dermatology hospitals and clinics as it enables dermatology procedures such as Botox and filler injections to be performed while causing little to no damage to the skin.
The company plans to gain a greater share of the market by developing a miniaturized version of the ‘needleless syringe’ for personal use, and this is expected to help further bolster Daejeon’s status as 'a leading biomedical city'.




 Interview With
Interview With
 City & Culture
City & Culture
 Food & Travel
Food & Travel
 Health & Wellness Tips
Health & Wellness Tips
 Hot Issue
Hot Issue

 Medical Technology
Medical Technology