Daejeon, Taking Leadership as Korea’s Biomedical City
Fostering some 300 bio startups with frontier technologies… Expected to emerge as Korea’s leading bio cluster…
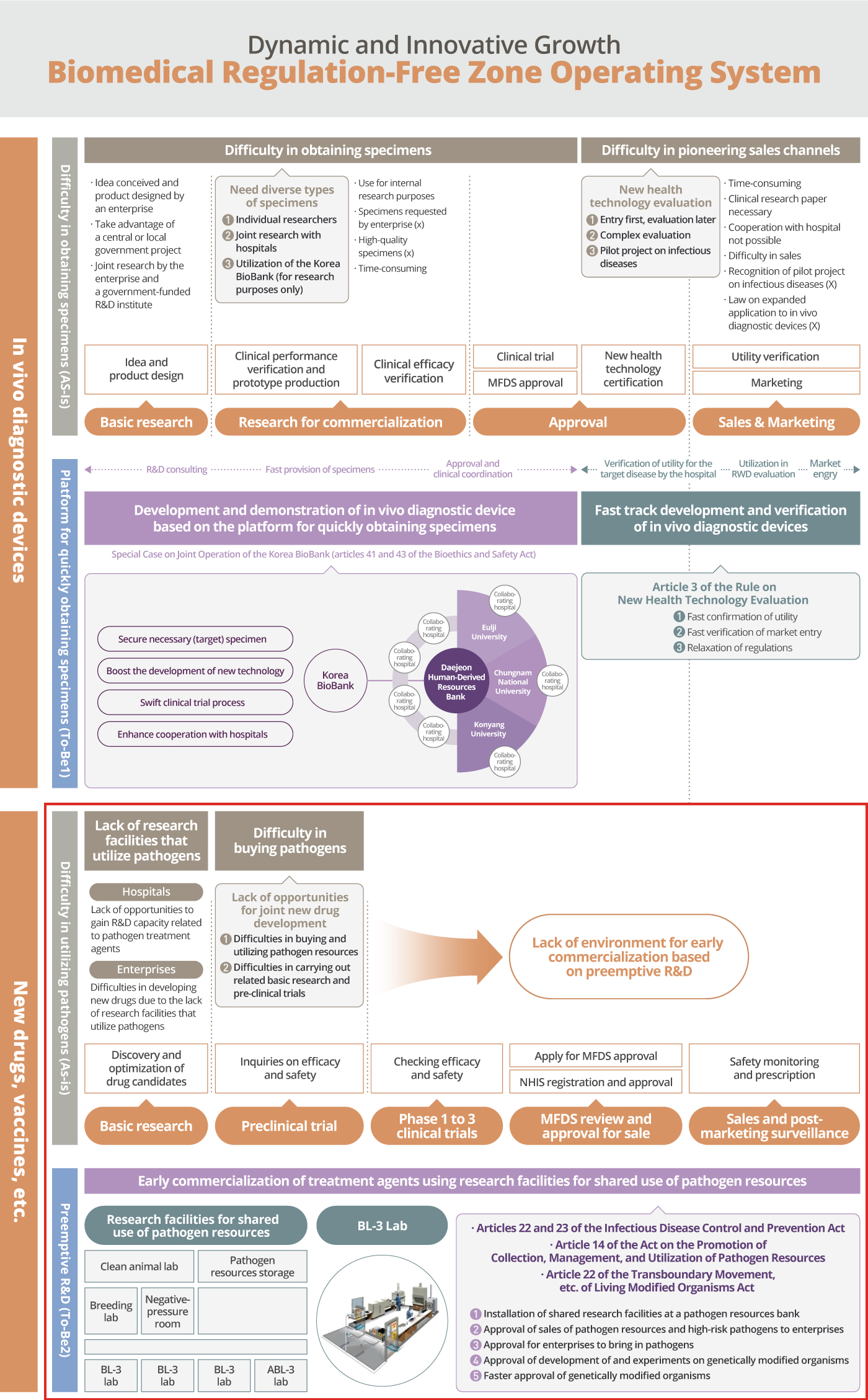
Daejeon has announced that the demonstration project for the commercialization of vaccines and treatment agents, based on the establishment and operation of R&D facilities for the sharing of pathogenic resources, had been chosen at the 3rd meeting of the Regulation-Free Zone Committee hosted under the supervision of the Ministry of SMEs and Startups.
This is expected to drive the momentum in the development of vaccines and treatment agents for infectious diseases by the bio companies in Daejeon. The project is expected to lower the barriers to entry for startups in the field of vaccine and treatment agent development and lead to the establishment of a one-stop support system for the bio industry in relation to the development of diagnostic kits, vaccines, and treatment agents for infectious diseases.
It is anticipated that the project will reduce the time it takes for bio startups to develop and launch vaccines and treatment agents for infectious diseases. Early product verification and accelerated market releases of high-quality products will help the Korean bio industry gain a competitive edge in the global market and in turn revitalize the local economy.
With the designation as the Biomedical Regulation-Free Zone, Daejeon is expected to attract some 30 biomedical enterprises, create 2,300 new jobs, and produce added value that is worth around KRW 216 billion.
An official from Daejeon Metropolitan City Government said, “We will do our best to turn Daejeon into a biomedical city, based on the designation as the Biomedical Regulation-Free Zone, in connection with the 2030 bio industry strategy of the Korea Research Institute of Bioscience & Biotechnology (KRIBB).”



 Interview With
Interview With
 Medical Technology
Medical Technology
 Food & Travel
Food & Travel
 Health & Wellness Tips
Health & Wellness Tips
 Hot Issue
Hot Issue
 City & Culture
City & Culture