
Due to the aging population, the increase in the incidence of chronic diseases, and the consequent increase in the diversity and complexity of surgical procedures, surgical robots are emerging as icons of the future medical industry. To develop surgical robots, this field requires high-precision and high-level technology. ROEN Surgical, Inc. is a manufacturer of medical surgical robots that conducts research, develops, and markets flexible surgical robots that can treat tumors and stones by entering the human body through natural orifices. It is a startup founded by CEO Dong-Soo Kwon and eight students in February 2018 based on 25 years of accaumulated medical robot research.
The 66th faculty startup at KAIST, the company is located in Jinri-gwan, KAIST
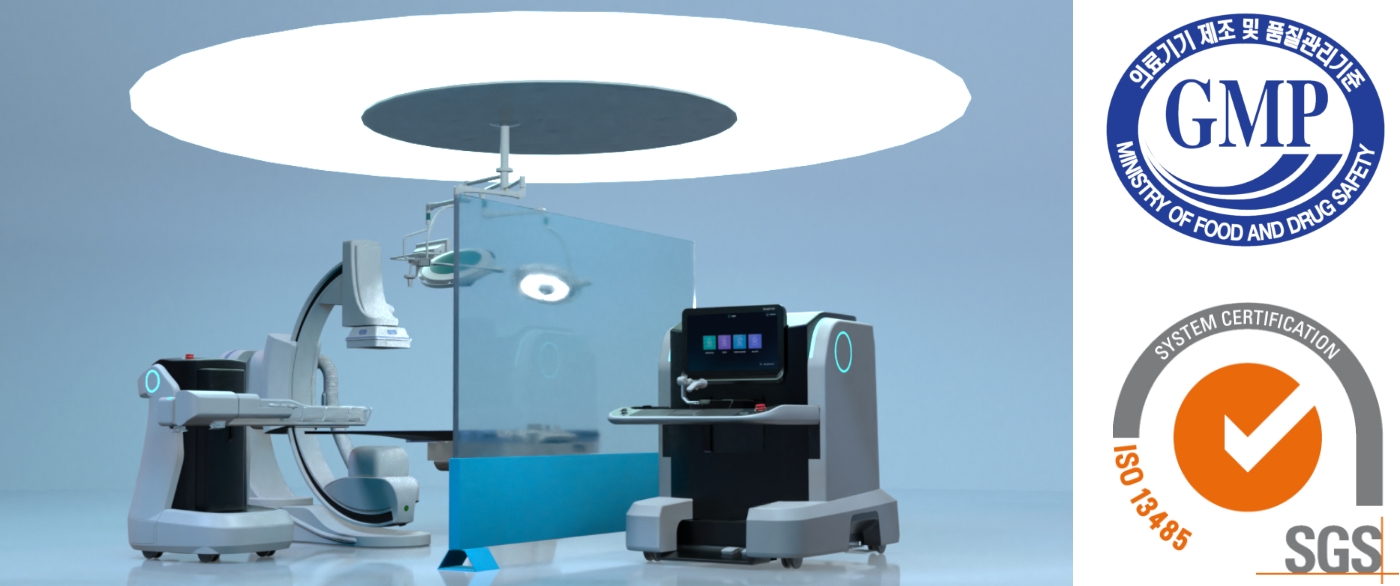
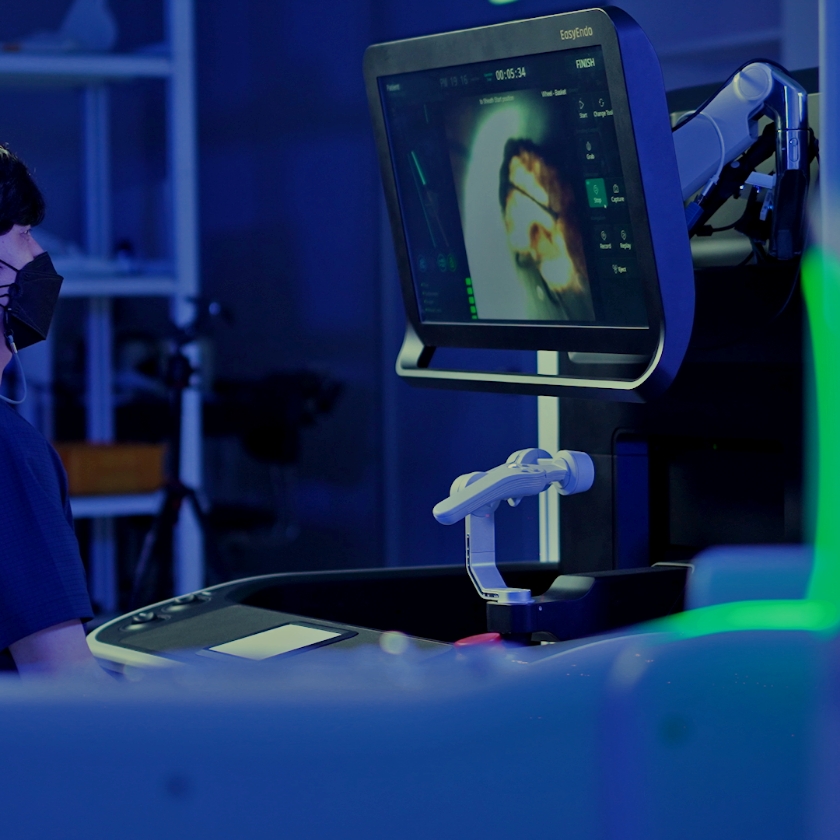
Since the introduction of laparoscopic surgery robots to compensate for the disadvantages of open surgery, surgery has often been performed using robots in recent years. Robotic surgery is convenient because the surgeon can perform surgery while seated, and it has the advantage of being able to zoom in on the surgical area, allowing for precise surgical operations and accelerating recovery, but the range of applicable procedures is still limited. Accordingly, Kwon Dong-soo, CEO of ROEN Surgical Inc., developed a surgical robot that can satisfy the needs of surgeons, hospitals, and patients by not only enhancing the convenience and stability of existing surgery but also minimizing damage to the affected area of the patient. As a result of combining a flexible ureteroscope with a surgical robot, Zamenix™ R, a kidney stone removal surgery robot with improved accuracy and stability, was born as the company's first product.
The robot for kidney stone removal, ZamenixTM R is equipped with a flexible ureteroscope and minimally invasively accesses the kidney through the urethra without skin incision or scarring, then breaks up and removes the stones. In comparison to kidney stone removal using a conventional flexible ureteroscope, this reduces surgeon fatigue and the difficulty of collaboration between surgeon and assistant, thereby simplifying the procedure and decreasing radiation exposure with a radiation shield barrier. From the patient's perspective, it is expected to reduce the risk of post-operative complications and decrease the patient's pain, thereby facilitating a speedy recovery.
In recognition of the superiority and originality of this technology, this product was designated the '17th Innovative Medical Device' by the Ministry of Food and Drug Safety on December 2, 2021. After successfully completing clinical trials with 47 participants in April 2022, the company obtained a manufacturing license on October 25, 2022, and ISO 13485 certification for our quality management system on November 2, 2022.
ROEN Surgical Inc. anticipated that if this product is is successfully applied to the medical field, convenience and precision will be enhanced in comparison to conventional kidney stone removal surgery. In addition, the technological level of the domestic surgical robot industry will be able to compete with that od multinational corporations, which will enable us to increase our presence in international markets like the United States and Europe.




 Specialized Medical Service
Specialized Medical Service
 Bio Technology
Bio Technology
 Health & Wellness
Health & Wellness
 City & Culture
City & Culture
 Hot Issue
Hot Issue
 Interview With
Interview With
 Medical Technology
Medical Technology
 City & Culture
City & Culture
 Food & Travel
Food & Travel
 Health & Wellness Tips
Health & Wellness Tips
 Hot Issue
Hot Issue